Sikkerhet
Informasjonen på denne siden er på engelsk.
The ADZYNMA safety profile was demonstrated in a Phase 3, open-label, crossover trial (NCT03393975), adverse events occurred in 71% of the patients with ADZYNMA. [2]
Informasjonen på denne siden er på engelsk.
The ADZYNMA safety profile was demonstrated in a Phase 3, open-label, crossover trial (NCT03393975), adverse events occurred in 71% of the patients with ADZYNMA. [2]

Hypersensitivity: [1]
Allergic-type hypersensitivity including anaphylactic reactions may occur. Patients should be informed of the early signs of hypersensitivity reactions including but not limited to tachycardia, tightness of the chest, wheezing and/or acute respiratory distress, hypotension, generalised urticaria, pruritus, rhinoconjunctivitis, angioedema, lethargy, nausea, vomiting, paraesthesia, restlessness, and may progress to anaphylactic shock. If signs and symptoms of severe allergic reactions occur, immediately discontinue administration of ADZYNMA and provide appropriate supportive care.

Immunogenicity: [1]
As with all therapeutic proteins, there is a potential for immunogenicity. Patients may develop antibodies to rADAMTS13 following treatment with ADZYNMA which could potentially result in a decreased response to rADAMTS13. If such antibodies are suspected and there is a lack of efficacy, consider other therapeutic strategies.
*List of excipients. Powder: Sodium chloride, Calcium chloride dihydrate, L-Histidine, Mannitol, Sucrose, Polysorbate 80 (E433). Solvent: Water for injections.
In a Phase 3, open-label, crossover trial (NCT03393975), no ADZYNMA-related serious adverse events were reported. [2]
The safety profile of ADZYNMA was evaluated in two studies: [1]
The most common adverse reactions reported in clinical studies were headache (31.5%), diarrhoea (17.8%), dizziness (16.4%), upper respiratory tract infection (15.1%), nausea (13.7%), and migraine (11%). [1]
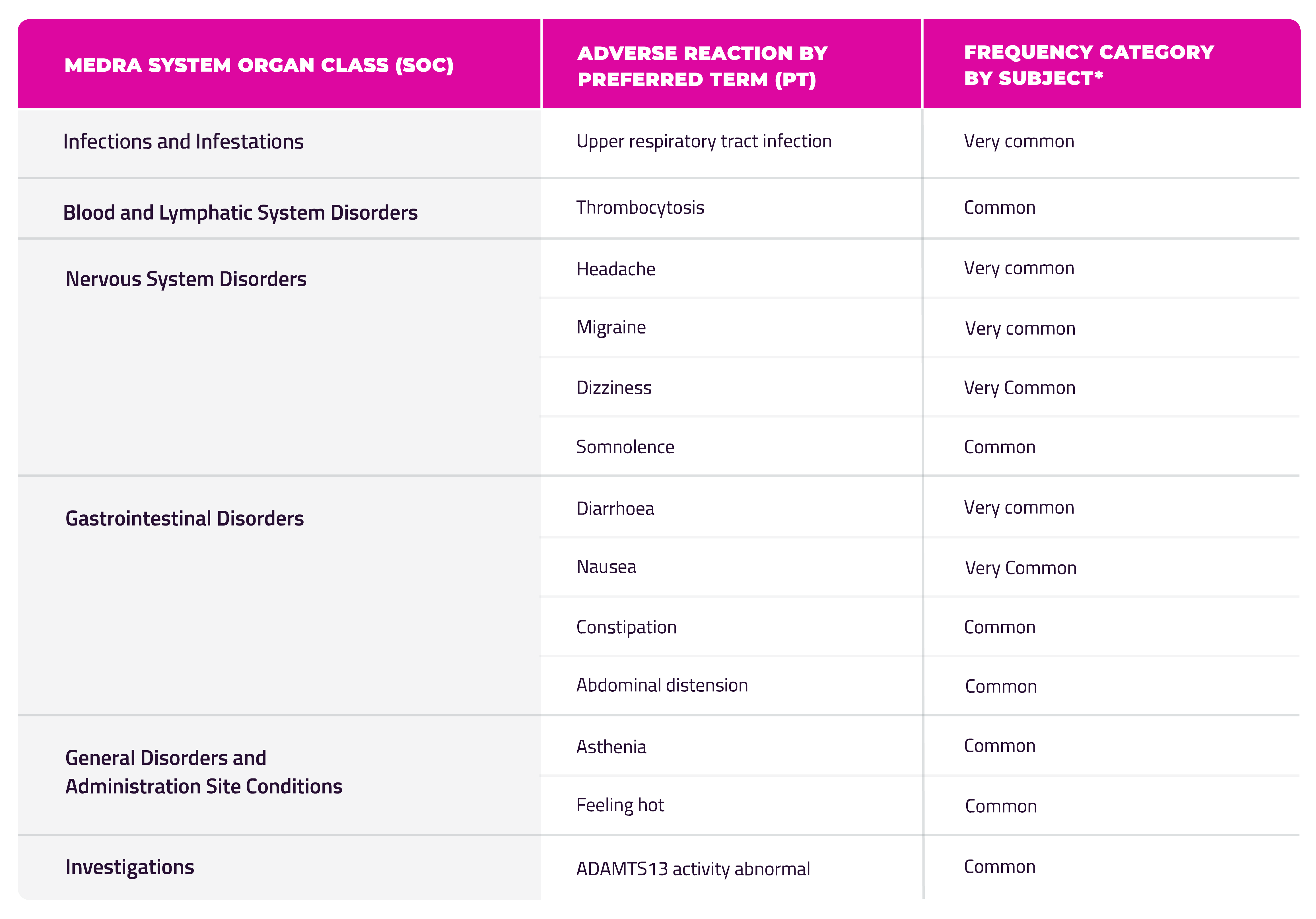

No patients receiving ADZYNMA had adverse events leading to treatment discontinuation or interruption. [2]
* Adverse reactions are listed above by MedDRA system organ class and by frequency. Frequencies are defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1 000 to < 1/100); rare (≥ 1/10 000 to < 1/1 000); very rare (< 1/10 000); not known (cannot be estimated from the available data). Within each System Organ Class (SOC), ADRs are presented in order of decreasing frequency. Within each frequency grouping, ADRs are presented in order of decreasing seriousness.
Acronyms
ADAMTS13, A disintegrin and metalloproteinase with a thrombospondin motifs 13
cTTP, Congenital TTP
CHO, Chinese hamster ovary
DNA, Deoxyribonucleic acid
eGFR, Estimated glomerular filtration rate
ERT, Enzyme replacement therapy
FFP, Fresh frozen plasma
FVIII, Factor VIII
IU, International unit rADAMTS13
rADAMTS13, Recombinant ADAMTS13
S/D, Solvent detergent SmPC
SmPC, Summary of product characteristics
TTP, Thrombotic thrombocytopenic purpura
VWF, Von Willebrand factor
1. ADZYNMA (rADAMTS13) EU Summary of Product Characteristics. August 2024
2. Scully M, et al. N Engl J Med. 2024;390(17):1584-1596