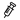No acute TTP event occurred during prophylaxis with recombinant ADAMTS13, whereas 1 patient had an acute TTP event during prophylaxis with standard therapy (mean annualised event rate, 0.05) [1], [2]
ADZYNMA pharmacology
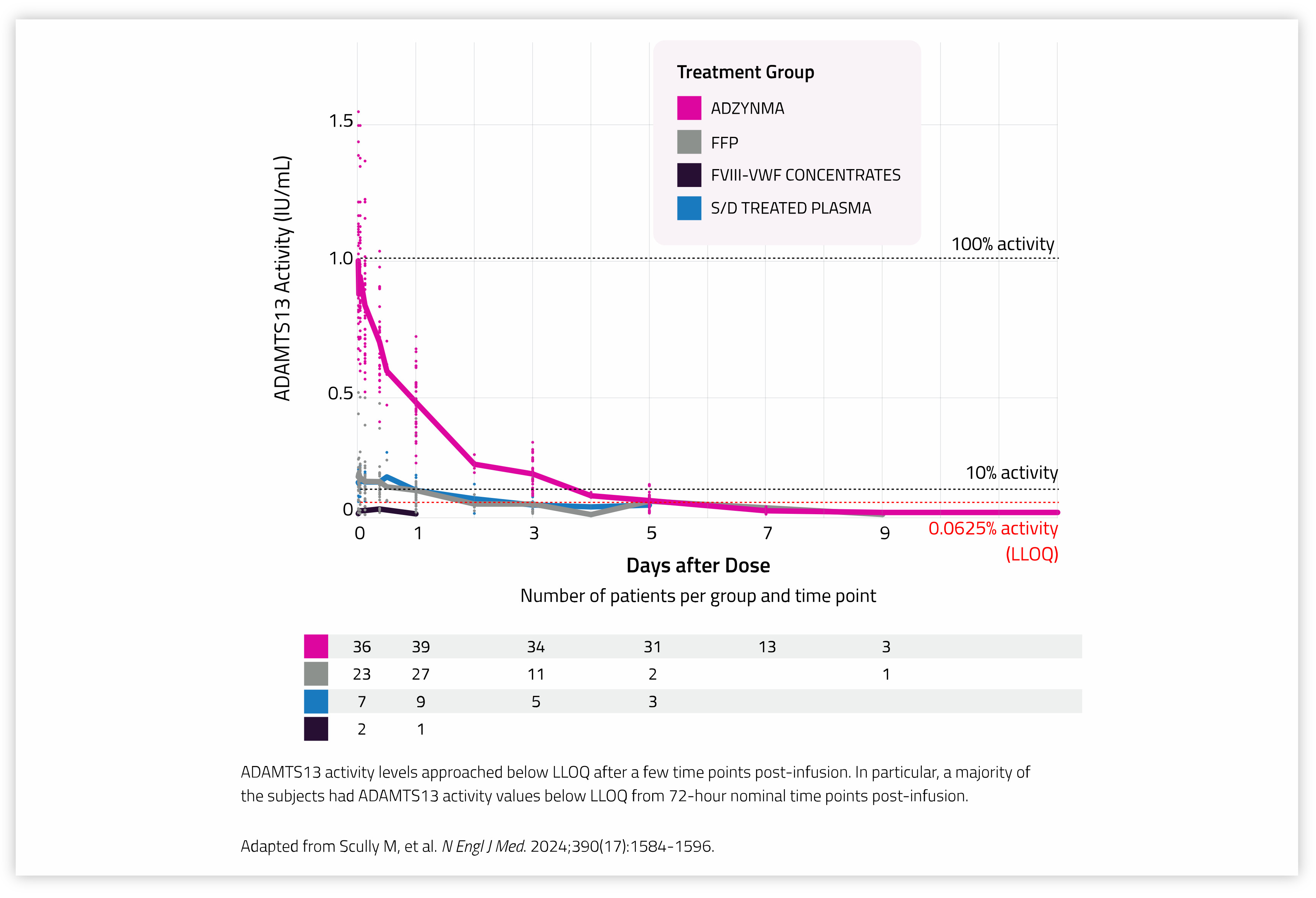
ADZYNMA IV administration at 40 IU/kg resulted in approximately greater than 5-fold higher ADAMTS13 activity exposures (Cmax, AUC, and duration above 10% ADAMTS13 activity) and lower variability when compared to plasma-based therapies.* [1]
✓ The mean maximum ADAMTS13 activity after recombinant ADAMTS13 treatment was 101%, as compared with 19% after standard therapy. [2]
✓ ADZYNMA IV administration at 40 IU/kg resulted in approximately greater than 5-fold higher ADAMTS13 activity exposures (Cmax, AUC, and duration above 10% ADAMTS13 activity) and lower variability when compared to plasma-based therapies.* [1]
✓ In a Phase 3, open-label, crossover trial (NCT03393975), the mean time with ADAMTS13 activity of ≥10% was 5.2 days after ADZYNMA administration vs 1.7 days after standard therapy. [1], [2]
✓ The prophylactic dose of ADZYNMA is 40 IU/kg of body weight, administered once every other week. The prophylaxis dosing frequency may be adjusted to 40 IU/kg of body weight once weekly based on clinical response. [1]
✓ Prophylactic treatment with ADZYNMA in the Phase 3, open-label, crossover trial (NCT03393975) resulted in a lower annualised incidence rate of composite cTTP manifestations (at least one of the following: thrombocytopenia, elevated LDH level, increased creatinine level, neurological symptoms, or abdominal pain) vs plasma-based therapy. [1], [2]
*The PK profile of ADZYNMA was determined based on clinical trial ADAMTS13 activity data analyses. Following single-dose intravenous administration of ADZYNMA at 5 IU/kg, 20 IU/kg, and 40 IU/kg to adults and adolescents, dose-related increases in individual ADAMTS13 activity were observed and reached a maximum at approximately 1 hour post-administration or earlier. [1]
ADZYNMA indication
ADZYNMA can be used prophylactically, and on-demand, to treat congenital TTP in children and adults.* [1]