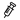QdengaTM er basert på et levende attenuert DENV-2-virus som utgjør den genetiske hovedkjeden for alle de fire viruskomponentene i vaksinen [1], [2], [3]
- QdengaTM ble utviklet ved bruk av rekombinante teknikker og inneholder levende svekket denguevirus [1], [2], [3]
- QdengaTM antas å replikeres lokalt og fremkalle nøytraliserende antistoffer mot denguesykdom som skyldes noen av de fire denguevirusserotypene [1], [2], [3], [4], [5]
- QdengaTM er utviklet for å aktivere flere armer av immunsystemet, inkludert bindende antistoffer, komplementbindende antistoffer, funksjonelle antistoffer mot ikke-strukturelt dengueprotein 1 (NS1) og cellemedierte immunresponser (CD4+, CD8+) og naturlige dreperceller [4], [5], [6], [7], [8], [9], [10]
- QdengaTM er utviklet for å blokkere et viktig virusprotein (NS1) som bidrar til patogenese [4], [8]
Immunresponser fremkalt av QdengaTM kan hjelpe med å beskytte pasientene dine mot denguesykdom: [2], [3], [4]
- Medfødte immunresponser (f.eks. interferon) [4]
- Adaptiv immunitet (f.eks. nøytraliserende antistoffer) [2], [3], [11]
- Cellemediert og humoral immunitet [4], [5]
Referanser
-
1. [SPC] QdengaTM Summary of Product Characteristics (12/2022)
-
2. [HUA03] Huang CY, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77(21):11436-47.
-
3. [OSO11] Osorio JE, Huang CY, Kinney RM, Stinchcomb DT. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine. 2011;29(42):7251-60.
-
4. [CHU15] Chu H, George SL, Stinchcomb DT, Osorio JE, Partidos CD. CD8+ T-cell Responses in Flavivirus-Naive
Individuals Following Immunization with a Live-Attenuated Tetravalent Dengue Vaccine Candidate. J Infect Dis. 2015;212(10):1618-28. -
5. [MIC21] Michlmayr D, Andrade P, Nascimento EJM, Parker A, Narvekar P, Dean HJ, et al. Characterization of the Type-Specific and Cross-Reactive B-Cell Responses Elicited by a Live-Attenuated Tetravalent Dengue Vaccine. J Infect Dis. 2021;223(2):247-57
-
6. [DeM22] DeMaso CR, Karwal L, Zahralban-Steele M, Dominguez D, Springer ZL, Kaiser M, et al. Specificity and Breadth of the Neutralizing Antibody Response to a Live-Attenuated Tetravalent Dengue Vaccine. J Infect Dis. 2022;226(11):1959-63.
-
7. [TRI22VACCINE] Tricou V, Gottardo R, Egan MA, Clement F, Leroux-Roels G, Sáez-Llorens X, et al. Characterization of the cell-mediated immune response to Takeda's live-attenuated tetravalent dengue vaccine in adolescents participating in a phase 2 randomized controlled trial conducted in a dengue-endemic setting. Vaccine. 2022;40(8):1143-51.
-
8. [SHA20] Sharma M, Glasner DR, Watkins H, Puerta-Guardo H, Kassa Y, Egan MA, et al. Magnitude and Functionality of the NS1-Specific Antibody Response Elicited by a Live-Attenuated Tetravalent Dengue Vaccine Candidate. J Infect Dis. 2020;221(6):867-77.
-
9. [NAS21] Nascimento EJM, Norwood B, Parker A, Braun R, Kpamegan E, Dean HJ. Development and Characterization of a Multiplex Assay to Quantify Complement-Fixing Antibodies against Dengue Virus. Int J Mol Sci. 2021;22(21).
-
10. [WAI19] Waickman AT, Victor K, Li T, Hatch K, Rutvisuttinunt W, Medin C, et al. Dissecting the heterogeneity of DENV vaccine-elicited cellular immunity using single-cell RNA sequencing and metabolic profiling. Nat Commun.2019;10(1):3666.
-
11. [BIS20] Biswal S, Borja-Tabora C, Martinez Vargas L, Velásquez H, Theresa Alera M, Sierra V, et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial. Lancet. 2020;395(10234):1423-33 and supplementary appendix.